One Step HSV Rapid Diagnostic test, to detect antibodies to HSV 1/2
,Gold colloidal,quickly and easily
Product Name:
One Step Herpes Simplex Virus Rapid Diagnostic test
One Step HSV Rapid Diagnostic test
Intended Use:
The Herpes Simplex Virus Rapid Test is a rapid qualitative lateral
flow test designed for the quantitive detection of IgM antibodies
to Herpes Simplex Virus (HSV) in human serum/plasma samples.
Summary:
HSV-1 is usually associated with infection in oropharyngeal area
and eyes, while HSV-2 causes mostly genital and neonatal infections
(5, 6), however, the tissue specificity is not absolute (7). HSV-2
can be isolated occasionally from the oropharynx and 5-10% of
primary genital infections may be caused by HSV-1. Infants infected
with HSV appear normal at birth, but almost invariably develop
symptoms during the newborn period (5, 8, 9). Neonatal HSV
infection may remain localized or become disseminated. Localized
infection may involve one or a combination of sites. These are
skin, eyes, mouth or the central nervous system. Disseminated
infection is manifested by pneumonitis, hepatitis, disseminated
intravascular coagulopathy and encephalitis. Of the infants with
neonatal HSV, about one half of those surviving will develop severe
neurological or ocular sequelae. A number of serological procedures
have been developed to detect antibodies to HSV. These include
complement fixation, indirect immunofluorescent antibody, plaque
neutralization, and ELISA (6, 8, 10). Antibody of the IgM class is
produced during the first 2-3 weeks of infection with HSV and
exists only transiently in most patients. Serologic procedures,
which measure the presence of IgM antibodies, help discriminate
between primary and recurrent infections, since IgM antibodies is
rarely found in recurrent infections. High affinity IgG antibodies
to HSV, if present in a sample, may interfere with the detection of
IgM specific antibody (9). High affinity IgG antibody may
preferentially bind to HSV-1 antigen leading to false negative IgM
results. Also, rheumatoid factor, if present, along with antigen
specific IgG, may bind to IgG causing false positive IgM results.
Both problems can be eliminated by deactivating IgG in the sample
before testing for IgM.
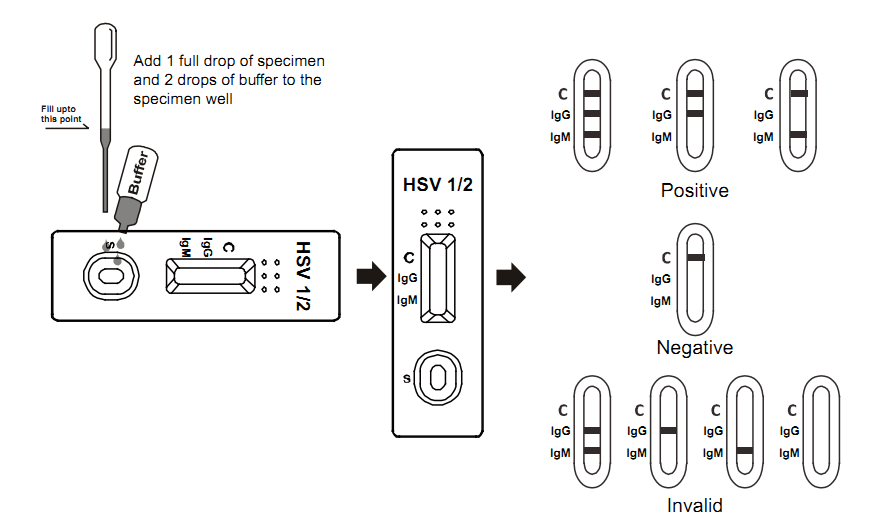
TEST PROCEDURE
1. Bring the pouched test device to room temperature(15-30℃) prior
to testing. Do not open pouch until ready
to perform the assay.
2. Remove the test device from the sealed pouch. Lay it on a flat,
clean and dry surface.
3. Use the pipette to draw and slowly add 1 drop of whole
blood/serum/plasma to the sample well.
4. Hold the buffer vertically and add 1 drop to the sample well.
/If using a pipette, change a new one to avoid
cross-contamination. Draw and transfer 2 drops of buffer to the
sample well.
5. Interpret test results within 10-15 minutes. Do not interpret
after 20 minutes.
Caution: The above interpreting time is based on room temperature range of
15-30℃. If your room temperature is significantly lower than 15℃,
then the interpreting time should be properly increased to 30
minutes.
INTERPRETATION OF RESULTS
Positive:
Two red lines are visible in the result window. The intensity of
the test line may be weaker or darker than that of the control
line. This still means a positive result.
Negative:
The control line appears in the result window, but the test line is
not visible.
Invalid:
If the control line does not appear in the result window, the test
results are INVALID regardless of the presence or absence of the
line in the test region.
PERFORMANCE CHARACTERS:
Table: HSV Rapid Test vs. EIA Test
Relative Sensitivity: 98.04% (89.55%-99.95%)* Relative Specificity: 98.33% (91.06%-99.96%)* Overall Agreement: 98.20% (93.65%-99.78%)* *95% Confidence Interval | | HSV Rapid Test | |
| + | - | Total |
| EIA Test | + | 50 | 1 | 51 |
| - | 1 | 59 | 60 |
| 51 | 60 | 111 |
NOTE: Insufficient specimen volume or incorrect procedural techniques
are the most likely reasons for the control line failure. Review
the procedure and repeat the test with a new device. If problem
persists, please contact your local distributor.
| ORIENT NEW LIFE MEDICAL CO., LTD. |
| Contact: | Jerry Meng |
| Email: | Jerry @ newlifebiotest .com |
| Tel. | +86 18657312116 |
| SKYPE | enetjerry |








